COOLANT COLOR IS NOT ALWAYS A TRUE INDICATOR OF ANTIFREEZE TYPE
Improper antifreeze mixtures could result in cooling
system damage.
If you check under the hood of your vehicle, you could find any one of a variety of coolant colors, especially if you own a non-North American car. But as the old cliche goes, "Don't judge a book by its cover." When it comes to antifreeze, it's the formula, not the color, which is most important.
The new coolant rainbow may create a potential for confusion and possible cooling system damage if antifreezes are improperly mixed. In the 14 NA domestic vehicle brands, green and orange are the traditional "factory fill" antifreeze colors. However, in the 22 non-NA vehicle brands, a wide spectrum of original equipment antifreeze colors exist, including various shades of green, yellow, pink, red and blue.
The likelihood of color confusion and unidentifiable mixtures is considerable among consumers and professional installers alike. The dye color used in coolant traditionally used to signify the type of antifreeze, but that's no longer the case. That's why it's important for vehicle owners to know what type of antifreeze is used in their cars' cooling system prior to having it serviced.
The best advice on deciphering what kind of antifreeze your cooling system uses is to check your owner's manual or contact the Agent. Basically there are two different types of antifreeze: silicone-silicate formulas, which contain the traditional rust and corrosion inhibitors and last an average of two years or 30,000 miles; and organic acid formulas, which are used in the new, extended-life antifreezes and rated to last five years or 100,000 miles. Each formula works differently to combat cooling system rust and corrosion and mixing the two could result in cooling system damage. However, there are also special alloy-safe coolants absolutely necessary to some models including the Plus 8.
Should you wish to simply flush your system, (recommended
every two years)..please consult the GoMoG Coolant directory.
COOLANTS REVISTED - an explanation of modern coolants
by Lorne Goldman (December 2018)
Automotive coolants are a necessity because not all of the energy produced by the engine is converted into actual work. Much of it expresses itself in unwanted heat. Only around one-third of heat produced by the energy in gasoline is converted into work that powers the car. Another third goes out the exhaust system and the remaining third is absorbed by the engine’s coolant. This last third of the heat is hopefully expelled into the air by passing through the car’s radiator, into those cute little rad fins and swept away by the air rushing through the rad. I jhave revisited this topic because coolants have been changing so quickly over the last decade. Also, newer cars, becuase of more stringent emission laws, are set to run at a heat much higher ever before. What was true 30 years or even 5 years ago, is no longer true now. In this area, be VERY wary of the advice of "oldtimers" and what they have" used for 50 years".
Coolant is designed to meet two goals.
1. FREEZING AND BOILING happen for two reasons. We add chemicals to increase its boiling point and (more effectively) lower its freezing point. Sadly, these chemicals and their effect diminishes over time. So poor or low quality coolant can freeze, blocking the liquid’s flow and preventing it from doing its job of dispersing your engine’s heat, possibly causing a nasty overheating situation, even if the air temperature is -25° Celsius. Frozen coolant can also expand to crack water pumps and radiators.
Modern coolant is designed to be mixed with equal parts of distilled water and provides freezing protection down to -40° Celsius. Oddly, undiluted coolant will freeze at roughly -15° Celsius as it’s the chemical reaction with water Additionally, coolant does NOT muchly increase the boiling point over water. which provide the frigid protection. It is the pressure, provided by the rad cap to the enclosed system, that provides most of that.
2. Most importantly, COOLANT ALSO PROVIDES CORROSION PROTECTION to the engine innards and rad. Corrosion has become more of a risk as many engines have become aluminium and coolants/water can carry electricity which acts as a corrosion catalyst. Those of us with aluminium rads (either fully aluminum or core only) have even more of a reason to use some of the newer coolants.
Coolant technology has vastly changed in the last 20 years. Today’s chemistry can assure your coolant a long lifespan with great protection. And many manufacturers cook up a few extra additives designed to prevent corrosion forming inside the cooling system..(most notably BMW). There are three basic types of coolant chemistries available for use in modern automobiles and, no, they are not always immediately identifiable by their colour.
Until about 20 years ago, choosing a coolant was easy – you bought the green stuff. Now there are three basic types of coolant:
Inorganic Acid Technology (IAT)
IAT coolant is green in colour and generally called ‘traditional’ coolant. It was the old-style, green coolant used by American and Asian applications. In its European manifestation, it was typically dyed blue. Its main fault are the additives for corrosion prevention tended to break down quickly, necessitating a full flush every couple of years. It is mostly used in cars manufactured in the US. Without the dye, naturally, this coolant is colorless and transparent. So the greenish or bluish tinge is added to help identify it. It contains sulfate and phosphate corrosion inhibitors, but no additives. The sulfate and phophate are hostile to aluminum and therefore hasten its corrosion. This is often made worse by an electrical charge or the addition of a aluminum radiator. It deteriorates QUICKLY. I do not recommend it but there are some newer formulation green coolants (i.e Prestone) that are safer than the cheap stuff and cool more efficiently.OAT coolant appeared in 1996 under GM’s Dex-Cool brand which is dyed orange. Other automakers have adopted it or a variant choosing to tint their new coolants amber, pink, blue and green. (CRAZY!!) OAT coolants form chemical bonds with metal surfaces in a car’s cooling system, forming coatings that are more stable than those of IAT coolants and making the coolant more efficient, cooling your engine more. OAT technology doesn’t use phosphates or silicate additive packages as in IAT coolants. Corrosion inhibitors such as sebacate and 2-ethylhexanoic acid are used instead. OAT won’t protect brass and copper components used in earlier model cooling systems. This type of additive package lasts much longer than those used in IAT coolant. They also degrade more slowly, often called "long-life" coolants, good for as much as five years or 240,000 km. Since 1996, I have been using a 50-50 mixture of GM OAT coolant to which I add a bottle of Water Wetter. I observed it lowered my coolant temperature 2C below standard (green) coolant in the same Morgan, on the same test run tack on the same day and ambient temperature.
| N.B. Despite extensive research, I have yet to find any coolant that lowers coolant temperatures for EVERYONE, too many factors individual factor involved. However, the new products mentioned above are vasty superior for engine corrosion protection and last much longer. I find it lowered my running temperatures (2C) over the manufacturer's (Land Rover) previous recommendation (the old green stuff). (they now use OAT as well (but colored blue) I found that a huge surprise. Water Wetter surprised me as well. It lowered my big bad V8 another 2C. Test done on the same day within an hour driving the same roads at the same RPM. |
| Coolant scientists are working towards an universal coolant. Watch this space. |
| Be sure not to combine any of the two modern coolants, neither with the
traditional stuff of OAT and HOAT, as the resultant mixture can turn into a soupy sludge. This
could clog a car’s cooling system and lead to all kinds of expensive
problems. If you are changing from one to the other, do a THOROUGH or PROFESSIONAL flush. |
WATERLESS COOLANTS (a warning)
by Lorne Goldman et al
On
the other hand, the goal of Waterless "coolants" is merely not-to-boil-over, not to properly cool the car engine.
Yet boiling over is the most important warning sign something is amiss
with your engine. But
cooling the engine is of secondary importance for a Waterless Coolant.
Therefore they make
absolutely no sense for a thinking person UNLESS they have a racing car
in UKA autocross compeition. Boiling over is not only a racing violation and reason
for disqualification, it is very dangerous for you and anyone on the
track. Coolant covered brakes, negates their effect and coolant makes
road surfaces super slippery.
| WATCHPOINT: Higher horsepower (and higher emissions) can be produced with lower fuel temperatures as this allows for a higher fuel-to-air mixture. However, lower temperatures do not produce as efficient a "burn" as higher temperatures. So a compromise is made between environmental regulations and power. However, it is a given that the higher the engine temperature, the lower the potential bhp. |
In the more than a century of the development of automobiles, the best cooling liquid found has been pure distilled water, (possibly with which should also be carefully used when mixing with coolants. (Distilled water is used as it less likely to carry electricity.) In
fact, water was used alone in the early automobile years, until it
was discovered that internal engine corrosion occurred. This
discovery lead to the invention of coolant liquids, that would
inhibit corroson and (mildly) increase the booling point of the
water to
prevent boil-overs. Boil-overs produce vapor which increases the volume
of the coolant and the pressure on the coolant system. Expansion tanks and pressure capsare
used to deal with this phenomena as the effect of traditional
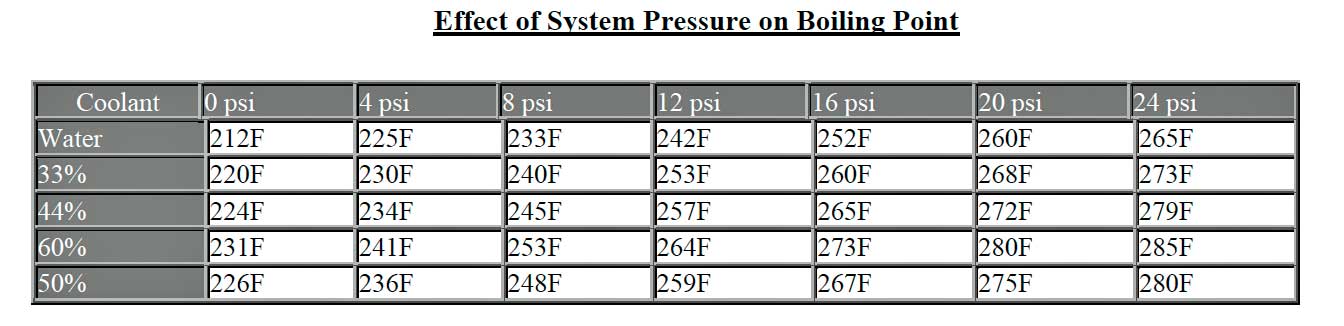
coolants alone is not enormous. The table below will help. You will not
that the relative cooling advantage of coolant declines with pressure.
However, this does not address the important protection for water/metal
reaction it provides along with a resistance to freezing.

As you can see from the table, a 50-50 coolant to water mixture with a 16psi cap will raise the boiling point to 267F or (130C)...which far beyond the temperature you or the engine's manufacturer ever want your engine to run at! Waterless Coollants allow your engine to run WAY overheated. Happily, at unhealthy, too hot temperatures, the same system will boil over, warning the driver that something is seriously amiss and to stop the car instantly. Coolant, boil overs are the best warning and owner has that they are causing damage to their cars. Using waterless coolants, like Evans, is analogous to stitching up a badly infected wound.
1. The cost of the waterless coolant is much higher. If you were to use a standard coolant or antifreeze in your vehicle, then the product cost would be around $20 per gallon. The cost of the waterless coolant from Evans, which is the primary supplier of this option, is about $40 per gallon. If you’re using a big-bore diesel engine with your truck, semi-trailer, or another large vehicle, the difference in price for your coolant needs may be $400+ if you choose the waterless option.RESULTANT CONCLUSION
Waterless coolants are designed to address the THE major symptom and warning signal of overheating and therefore engine and or coolijng system malfunction. They are not designed to improve cooling, but merely not boil over! And though they have excellent anti-corrosion properties (why not, they do not contain water?), they make no automobile sense. Owners regularly open up their engines to heat damage by using Waterless coolants. From an angry expert, (excuse his language he is VERY angry...:D). He later apologizes in another video after Evans threatened him with a baseless legal prosecution, a common legal tactic these days. One no longer has to be right in the eyes of a judge and jury. One simply wins by swamping one's opposition with legal fees. Happily I am immune to such tactics.
| N.B. There is only one activity in which I can see a use for waterless coolants and that would be American autocross. This is a sport that puts and enormous stress on a automobile for a short period of time and creates massive of amounts of short-term heat. Boilovers are common..which ruins the track for other competitors. Waterless coolants would be appropriately used here. HOWEVER, race tracks prohibit Evans products because they are flammable and slippery when spilled. |
Here is an excellent analysis of Evans Waterless Coolant done by a respected US laboratory:
|
EVANS WATERLESS COOLANT: OVERVIEW OF RESEARCH RESULTS Copyright 2012 Applied Chemical Specialties, Inc. Executive Summary by GoMoG, Evans and No-Rosion Evans offers several different iterations of their waterless coolant products. Each is 100% glycol. Some are 100% propylene glycol, and others are a mix of propylene glycol and ethylene glycol. The premise of their marketing is that, by excluding water from coolant, certain benefits can be achieved. Some of their advertised claims are: little to no pressure change during heat/cool cycles, less corrosivity, extended coolant life, less nucleate boiling, greater heat transfer, and improved performance. In our research, we evaluated each of these claims. CORROSION: We ran Evans waterless coolant through ASTM D1384 tests, and compared the weight losses due to corrosion (in milligrams) to that of No-Rosion for each of the metals tested:
* A negative weight loss indicates a weight gain. The product provides very good overall rates of corrosion protection, and passed ASTM D1384. The only concerns were: (a) the relatively high rate of corrosion for solder, and (b) the net gain in weight on aluminum. Inspection of the aluminum test coupon indicated inhibitor deposition from the Evans product. In a cooling system, this can cause problems. Inhibitor deposition causes hot-spots to develop on metal heat exchange surfaces. This can cause granular fatigue in aluminum radiators, and result in stress cracks and failures, depending on the thickness of the metal. FIRST USAGE AND CONVERSION It is important to note that this level of corrosion protection can only be achieved if the coolant consists of 97%-100% Evans coolant. If only 3% or more of coolant previously used in the system remains, the corrosion resistance of Evans coolant is lost. When this happens, water combines with the glycol in the Evans coolant to form glycolic acid. The result is reduction in coolant pH, and corresponding corrosion problems. It can prove problematic to fully remove 97%+ of coolant from a system. But doing so is mandatory in order to meet the Evans conversion requirement. It is a difficult, tedious process. Engine block frost plugs must be removed, the radiator must be disconnected, hoses evacuated, etc. In our testing, when we followed the Evans procedure for complete removal of coolant for our various test vehicles, the average observed removal rate was 94%. This would not be acceptable for conversion to the Evans products. To aid in this process, Evans sells a conversion fluid that can be used to facilitate more effective removal of previous coolant. It costs $34 per gallon. In most systems, one gallon is enough. But larger systems will require two gallons. Evans also has a list of authorized conversion centers, where vehicles can be taken, and mechanics perform the conversion process for you. We found typical conversion (updated 2022) costs $320 if done by the onwner and $450 if expert labor is added, plus a minimum of one gallon of conversion fluid at a cost of $34 per gallon.
When we followed Evans
directions for conversion, and did it ourselves, we were able to
successfully achieve the required 97%+ coolant removal in only about 60% of our
test vehicles. EFFECT ON CYLINDER HEAD TEMPERATURES VERSUS NORMAL COOLANT AND DISTILLED WATER After proper conversion to the Evans products, the average temperature of engine cylinder heads increased by 115-140oF, versus running with No-Rosion and water. The reason for hotter cylinder heads relates to the specific heat capacity of these different fluids. Water has a specific heat capacity of 1.00. It transfers heat more effectively than any other fluid, and is therefore used as the reference fluid in the scientific measure of specific heat capacity. Comparatively, the specific heat capacity of the various glycol solutions in the Evans products ranges from 0.64 to 0.68. So they conduct roughly half as much heat as does water, or water with No-Rosion. (No-Rosion does not alter the specific heat capacity of water.) Cylinder head temperatures of 115-140oF hotter with the Evans products translates to a stabilized bulk coolant temperature increase of 31-48oF, as compared to No-Rosion and water. As case in point, conversion of a Chevrolet LS-1 engine from No-Rosion and water to Evans Waterless Coolant resulted in an increase of 128oF at the cylinder heads. We saw a stabilized bulk coolant temperature of 192oF with water and No-Rosion, and 236oF with the Evans product. So the temperature increased by 44oF after converting to the Evans product. By having engine cylinder head temperatures 128oF hotter with the Evans product, a number of performance setbacks were observed: (1) the octane requirement was increased by 5-7 numbers, (2) the computerized ignition system retarded timing by 8-10o to avoid trace knock, (3) horsepower was correspondingly reduced by 4-5%, as confirmed on a chassis dyno. In our pre-1970s test vehicles, we also saw evidence of increased recession rates of non-hardened valve seats. When cylinder head temperatures are elevated to this degree, brinelling damage can occur. This is a process in which the metal seat softens due to heat that is beyond what it was originally designed to tolerate. Recession therefore occurs at an accelerated rate. Valve seat brinelling is seen in engines of vehicles built prior to the early 1970s, after they have been allowed to run too hot, for too long. Conversion to Evans products also requires reprogramming of ECUs in modern vehicles with electric fans. Most vehicle ECUs are programmed to turn the fan on at a coolant temperature of 200-210oF, and turn the fan off at 180-190oF. Because engines run so much hotter with Evans coolant, the ECU must be reprogrammed to an Evans-recommended turn-on temperature of 230oF, and an Evans-recommended turn-off temperature of 215oF. Without reprogramming the ECU, the fans would run continuously. Evans advertises a number of performance benefits in the area of reduced coolant nucleate boiling. In our research, we found that with proper conversion to the Evans product, its elevated boiling point did yield a 46% reduction in localized cylinder head nucleate boiling. However, even with this reduction in nucleate boiling, there were no observable enhancements in engine performance. This was due to the fact that the specific heat capacity of the 100% glycol coolant was not sufficient enough to translate into any meaningful temperature reduction. Comparatively, when used in straight water coolant, the high cloud point surfactants in No-Rosion achieve a 39% reduction in the size of localized nucleate bubbles. Smaller bubbles release quicker from the hot surface of the cylinder head, resulting in enhanced overall contact with the metal. Because water has a higher specific heat capacity than glycol, it is better able to translate this into meaningful temperature reduction. For this reason, No-Rosion achieves a net reduction in cylinder head temperatures, versus a net increase in cylinder head temperatures when Evans products are used. Cylinder head temperatures in our test engines ranged from 650oF to over 980oF. The Evans products have boiling points in the range of 369-375oF at 0 psi pressure. Straight water coolant with No-Rosion has a boiling point of 250oF at 15 psi. The interface between the cylinder head and engine coolant is the location of nucleate boiling. It does not matter whether coolant has a boiling of 375oF, or 250oF. Either way, nucleate boiling occurs. The fact that Evans coolant has a boiling point that is 125oF higher than water is not enough to completely prevent nucleate boiling. The only way this could be achieved would be through the use of coolant having a boiling point higher than the cylinder head temperatures, in the range of 650-980oF. (As an interesting side note, research is currently underway regarding the efficacy of glycerine as engine coolant. It’s extremely high boiling point of 554oF may offer benefits for future cooling applications.) It is important to realize that straight water has a high surface tension of 72 Dynes/cm2. When added at the proper dose, No-Rosion reduces the surface tension of water to 26 Dynes/cm2. Through this reduction in coolant surface tension, No-Rosion has the ability to alter the localized dynamics of heat exchange in cylinder heads, despite the fact that water has a lower boiling point than glycol. Comparatively, Evans coolants have surface tension in the range of 36-44 Dynes/cm2. In their advertising, Evans makes the claim that Evans NPG Coolant can maintain a substantially vapor free liquid to metal contact (nucleate vapor only) at all coolant temperatures and engine loads. In our research, we did not find this to be an accurate statement. As already referenced, we did observe a reduction of nucleate boiling with the Evans product. But we did not observe a substantially vapor free condition of nucleate boiling, as advertised by Evans. This was confirmed in laboratory simulations, utilizing an electric heat source that produced metal temperatures in the range 650-980oF. Further contributing to cylinder head temperature elevation is the fact that Evans waterless products are considerably more viscous than water, or a 50/50 mix. At operating temperatures, water, and water with No-Rosion, has a viscosity of 0.28 cp. (No-Rosion does not alter the viscosity of water.) A 50/50 mix has a viscosity of 0.70 cp. The Evans products have viscosities of 2.3 to 2.8 cp. In other words, Evans waterless products are almost 10 times more viscous than water coolant, and 3-4 times more viscous than a 50/50 mix. This creates significant drag on water pumps. OEM auto manufacturers design water pumps for the viscosity of a 50/50 mix. In our research, we observed a 20-25% reduction in coolant flow through radiator tubes when Evans waterless products were used. This is a direct result of Evans products higher viscosity. As coolant flow rates through radiator tubes drop, the ability of coolant to transfer heat via the radiator has a corresponding drop as well. Coolants decreased ability to transfer heat at lower flow rates is a result of the Second Law of Thermodynamics, as best expressed in the following the equation: Q = M x Cp x ΔT Where: Q is the heat load M is the mass flow rate of coolant Cp is the specific heat capacity of coolant ΔT is the change in temperature of coolant in the radiator Apparently in recognition of how their products negatively impact coolant flow rates as a result of their high viscosity, Evans now sells high volume water pumps for various engines, to include the Chevrolet LS1/L6. These pumps provide 20% more flow that OEM units, which would be almost enough to overcompensate for the greater pump effort required to move their considerably more viscous coolant fluids. There is speculation that, when OEM water pumps are used with viscous Evans waterless products, water pump life span could be reduced, and result in a greater frequency of water pump failures. Additional testing would be necessary in order to validate this. There is also speculation that cylinder head temperature increases of 115-140oF as a result of using 100% glycol coolant may cause warping and related damage to cast iron heads in some engines. OEM engines are designed to be run at temperatures that are consistent with what is produced using coolant consisting of a 50/50 mix. The higher temperatures produced by 100% glycol coolant could increase the frequency of cast iron head damage. Again, additional testing would be necessary in order to validate this. Because Evans waterless products are 100% glycol, they are slippery when spilled or leaked onto pavement. Assuming a baseline friction co-efficient reference of 1.00 for dry pavement, the friction co-efficient of water, and water with No-Rosion, is 0.65. (No-Rosion does not appreciably alter the friction co-efficient of water, when used at the proper dose.) The friction co-efficient of Evans products is 0.16. Evans products are 4 times more slippery than water. Race tracks now ban the use of engine coolant that contains ANY glycol. Instead, they require engines to run straight water coolant. This is one of the reasons why the Evans products can not be used in the engines of vehicles that are operated on a race track. The other reason that Evans products are prohibited at race tracks is that they are flammable. They have flash points in the range of 225-232oF. This means that if Evans coolant were released at or above the flash point, it could ignite. Because we observed coolant temperatures in this range during actual operating conditions, this is a real risk. On a comparative basis, straight water with No-Rosion has no flash point, and is not flammable at any temperature. The cost of Evans waterless coolant is about $230 for an average 4 gallon cooling system. If you were to pay an authorized Evans conversion center to perform it for you, it costs another $150-$180 in labor, and $34 for the conversion fluid. So the do-it-yourselfer will pay a total of about $264. Consumers who have the shop do it for them will pay $439. On a comparative basis, water is free. No-Rosion costs $10.00 per bottle at retail. The proper dose of No-Rosion for straight water coolant requires two bottles, at a total cost of $20.00. Are there engine cooling systems that will benefit from the physical properties of Evans waterless coolant? Absolutely. As a case in point, we have worked with a car collector who owns a 1931 Rolls-Royce Phantom II. It is powered by a 12-cylinder, Rolls-Royce Merlin aircraft engine, taken from a WWII P51 Mustang. The engine displaces 1,649 cubic inches, and creates an estimated 1,100 horsepower. Because this engine was originally designed to be operated in an airplane that flies at altitude, where the air is very cool, it has some significant cooling challenges when used in a vehicular application. The cooling system is essentially non-pressurized. So water coolant will boil at only 212oF, instead of the 250oF that it would boil at if the system were pressurized to 15 psi. Using water coolant results in boiling and engine overheating. This is the perfect application for Evans waterless coolant because of its high boiling point, even at zero pressure.
But how many of us drive a
car with a 1,100 horsepower Merlin WWII airplane engine taken from a P51
Mustang? Conversion costs of $259 if you do it yourself, or over $400 if you pay a shop to do it. 97%+ removal of all previous coolant is mandatory in order to prevent corrosion. Inhibitor deposition occurs on aluminum surfaces, which could cause issues in some radiators. Engines run 115-140oF hotter (at the cylinder heads) with Evans products. Stabilized coolant temps are increased by 31-48oF, versus straight water with No-Rosion. Reprogramming ECU fan temp settings is mandatory to prevent the fan from running continuously. Specific heat capacity of Evans waterless products ranges from 0.64 to 0.68, or about half that of water. Engine octane requirement is increased by 5-7 numbers. Computerized ignition must retard engine timing by 8-10o to prevent trace knock. Engine horsepower is reduced by 4-5%. Accelerated recession of non-hardened valve seats in older engines is possible, due to brinelling. Viscosity is 3-4 times higher than what OEM water pumps are rated to accommodate. Coolant flow rate through radiator tubes is reduced by 20-25% due to the higher viscosity. |
In short, not even EVANS suggests that it will better cool the car.