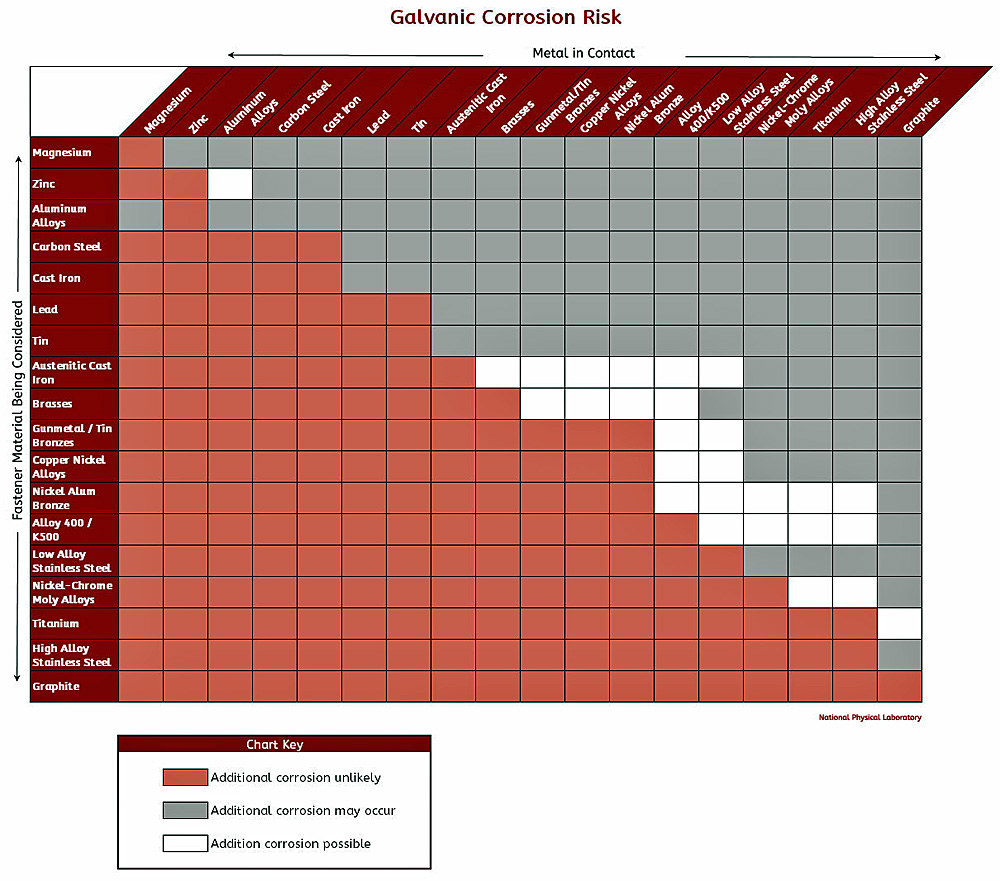
Galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Metals close to one another on the chart generally do not have a strong effect on one another, but the farther apart any two metals are separated, the stronger the corroding effect on the one higher in the list. This list represents the potential available to promote a corrosive reaction, however the actual corrosion in each application is difficult to predict. Typically, the presence of an electrolyte (eg. water) is necessary to promote galvanic corrosion. Please see the chart below. "The Laws of Nature Always Win".
